Structure of Atom
Presentations | English
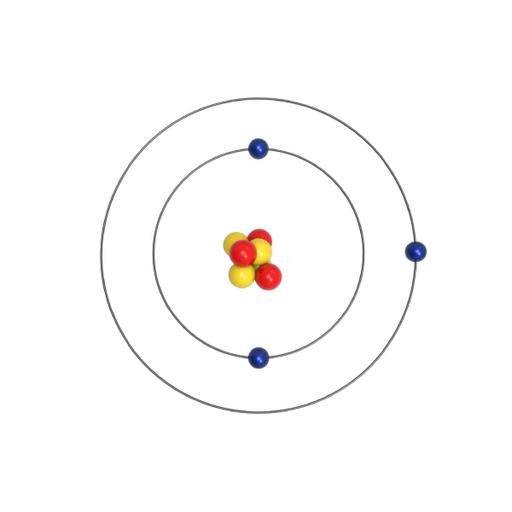
How can we explain the Structure of an Atom? An atom is the smallest unit of ordinary matter that forms a chemical element. It is the smallest unit into which matter can be divided without the release of electrically charged particles. The basic structure of an atom involves a nucleus and orbiting electrons. An atom is a complex arrangement of negatively charged electrons arranged in defined shells about a positively charged nucleus. This nucleus contains most of the atom's mass and is composed of protons and neutrons (except for common hydrogen which has only one proton). All atoms are roughly the same size. Atoms consist of three basic particles: protons, electrons, and neutrons. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The outermost regions of the atom are called electron shells and contain electrons (negatively charged).
15.50
Lumens
PPTX (62 Slides)
Structure of Atom
Presentations | English
