S and P Block Elements
Presentations | English
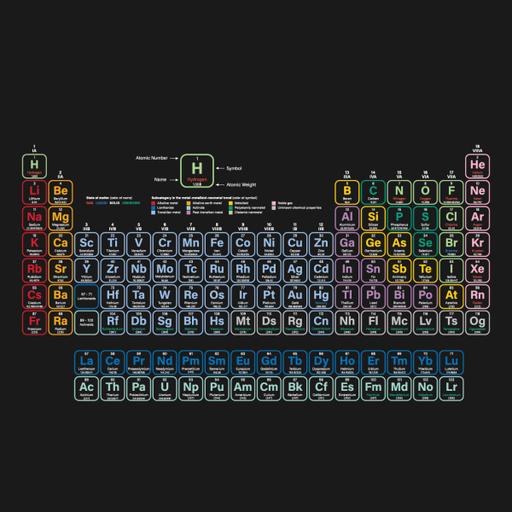
S and P block elements are chemical elements found in the periodic table of elements. They are also called Typical Elements to distinguish them from the transition and inner transition series. The main difference between S and P block elements is that the valence electrons of the S block elements are in the S orbital whereas the valence electrons of the P block elements are in the P orbital. The total number of S block elements is 14 (Group IA and IIA). The elements are soft with low boiling and melting points. In P-block elements the last electron enters the outermost P orbital. As we know that the number of P orbitals is three and, therefore, the maximum number of electrons that can be accommodated in a set of P orbitals is six. Consequently there are six groups of P–block elements in the periodic table numbering from 13 to 18. The presentation gives in-depth knowledge on the topic.
38.25
Lumens
PPTX (153 Slides)
S and P Block Elements
Presentations | English
