Periodic Classification of Elements
Presentations | English
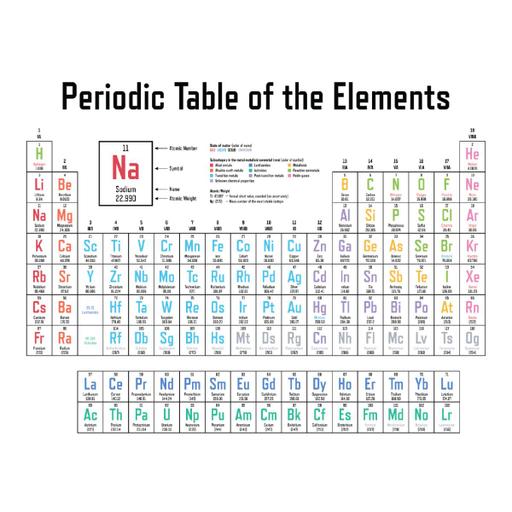
The periodic table is a tabular method of displaying the elements in such a way, that the elements having similar properties occur in the same vertical column or group. In 1913, Henry Moseley showed that the atomic number of an element is a more fundamental property than its atomic mass. The physical and chemical properties of elements are the periodic function of their atomic number. Modern periodic table is based on atomic number of elements. Atomic number (Z) is equal to the number of protons present in the nucleus of an atom of an element. Modern periodic table contains eighteen vertical columns known as groups and seven horizontal rows known as periods. On moving from left to right in a period, the number of valence electrons increases from 1 to 8 in the elements present. On moving from left to right in a period, number of shell remains same. All the elements of a group of the periodic table have the same number of valence electrons.
15.50
Lumens
PPTX (62 Slides)
Periodic Classification of Elements
Presentations | English
