Hydrogen Bonds in Water and Ice
Presentations | English
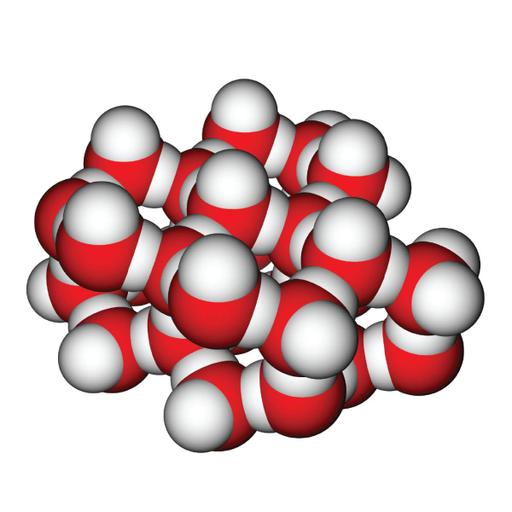
Each water molecule in ice forms an ice crystal when it is hydrogen linked to four other water molecules. This configuration is required to provide the highest degree of Hbonding in a homogeneous, stretched crystal lattice, resulting in larger ice structure openings. Each molecule of liquid water is H-bonded to 3.4 other water molecules. Because of the rapid thermal movements of molecules, these Hbonds are frequently produced and broken, yet hydrogen bonds in ice remain permanent. As ice is the strong shape of water and it has more hydrogen bonds than water, since it's oxygen particles are absolutely tetrahedrally situated and each oxygen is hydrogen reinforced by four neighbouring oxygen particles. Ice, like all solids, has a well-defined structure, with four H2O molecules surrounding each water molecule. Two of these are hydrogen-bound to the centre H2O molecule's oxygen atom, and each of the two hydrogen atoms is similarly connected to another H2O molecule.
25.25
Lumens
PPTX (101 Slides)
Hydrogen Bonds in Water and Ice
Presentations | English
