Atomic Structure and Periodicity
Presentations | English
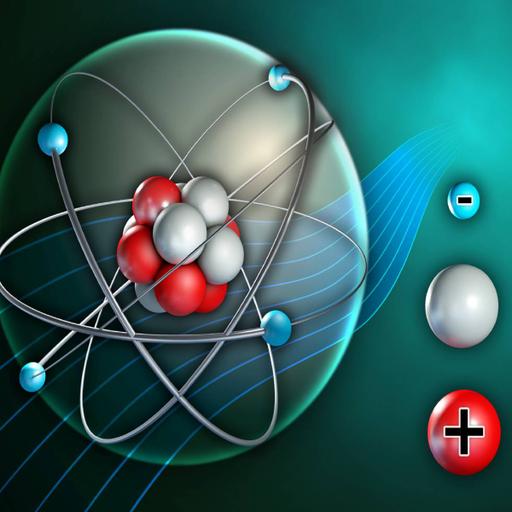
Atomic Structure and Periodicity presents basic atomic theory. The structure of the Periodic Table as we know is based on sound principles. Atomic structure refers to the structure of an atom comprising a nucleus (centre) in which the Protons (positively charged) and Neutrons (neutral) are present. The negatively charged particles called Electrons revolve around the centre of the nucleus. In the context of chemistry and the periodic table, periodicity refers to trends or recurring variations in element properties with increasing atomic number. Periodicity is caused by regular and predictable variations in element atomic structure. Periodic trends arise from the changes in the atomic structure of the chemical elements within their respective periods (horizontal rows) and groups in the periodic table. These laws enable the chemical elements to be organized in the periodic table based on their atomic structures and properties. Due to the periodic trends, the unknown properties of any element can be partially known. The presentation gives better understanding of the topic.
21.75
Lumens
PPTX (87 Slides)
Atomic Structure and Periodicity
Presentations | English
